
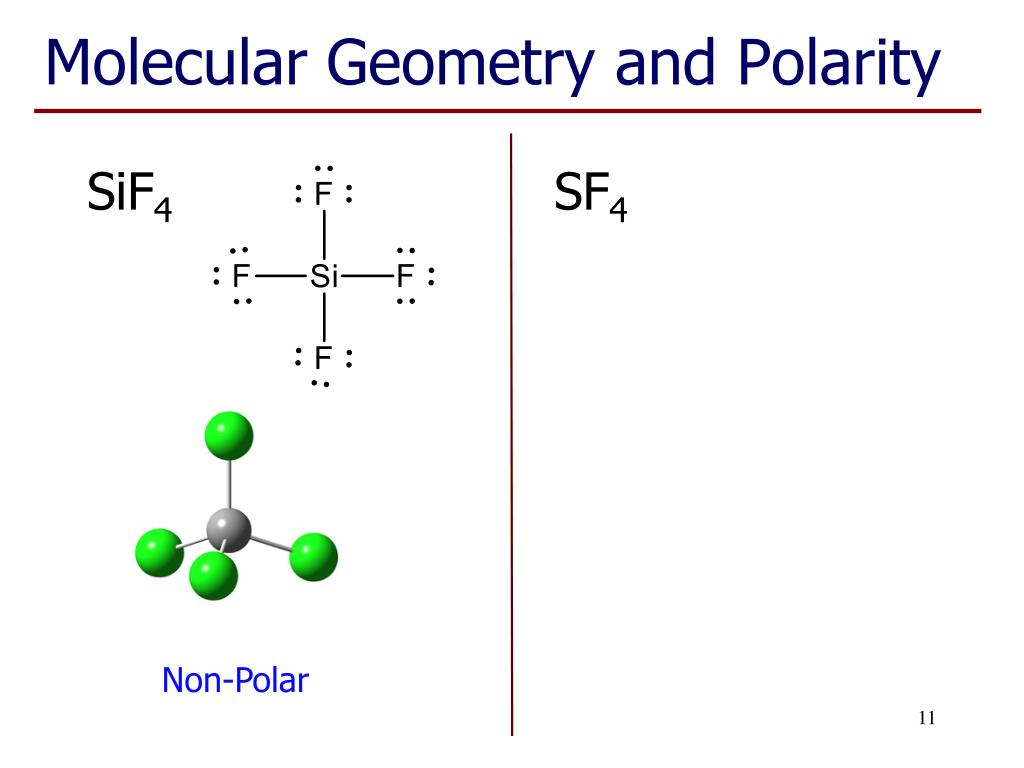
First draw the Lewis electron dot diagram for water and determine its molecular shape. The first two steps remain the same as the tail-to-head method: 1. Let’s examine this method again for a molecule of water. An alternative method to determine the vector sum of dipole arrows is known as the vector component method. Now superimpose the net molecular dipole arrow onto the molecule. Draw a new line connecting the tail of the first vector. VSEPR theory predicts a linear molecule: The CO bond is considerably polar. The shape of a molecule is based on the number of. To determine if this molecule is polar, we draw the molecular structure.
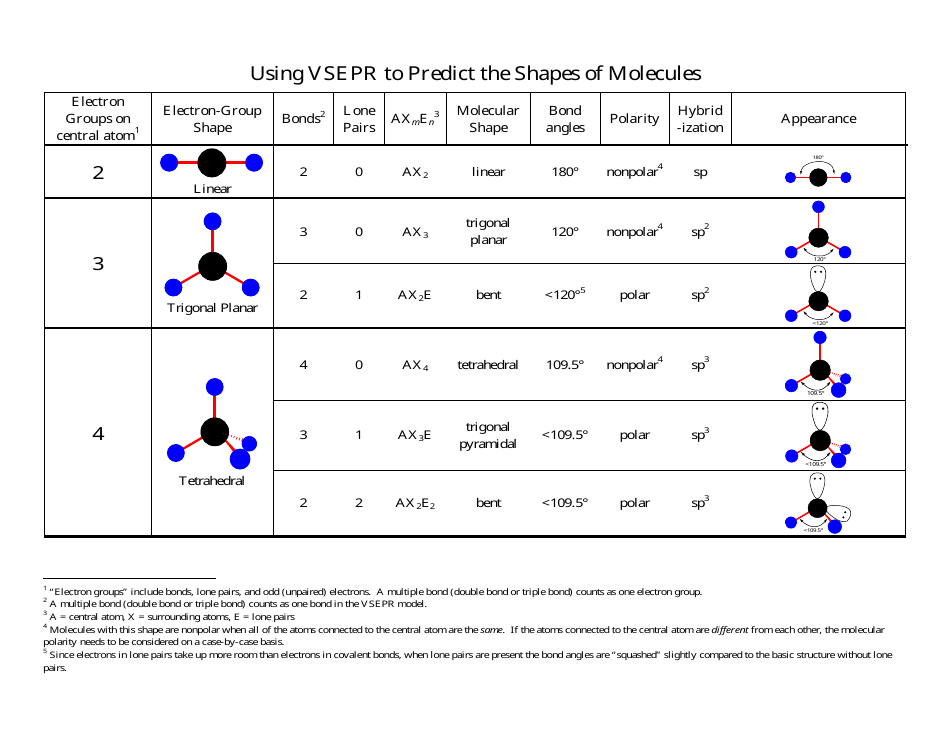
Draw in dipole arrows for all polar covalent bonds, starting the arrow at the more electropositive atom, and ending at the more electronegative atom. The two molecular geometries in which the molecule will remain polar are tetrahedral and square planar. Water has four electron groups, but only two atoms attached to the central atom so it is bent. Let’s examine this method for a molecule of water. A the central atom, X an atom bonded to A, E a lone pair on. One method to determine the vector sum of dipole arrows is known as the tail-to-head method. Therefore the molecular polarity is the vector sum of the individual bond dipoles. Each bond’s dipole moment can be treated as a vector quantity, having a magnitude and direction. In this video we’ll use VSPRE Theory to practice the rules for identifying the major molecular geometries, including bond angles. The overall polarity of molecules with more than one bond is determined from both the polarity of the individual bonds and the shape of the molecule. Table 9.4 Summary of Molecular Shapes Number of Electron Groups on Central Atom
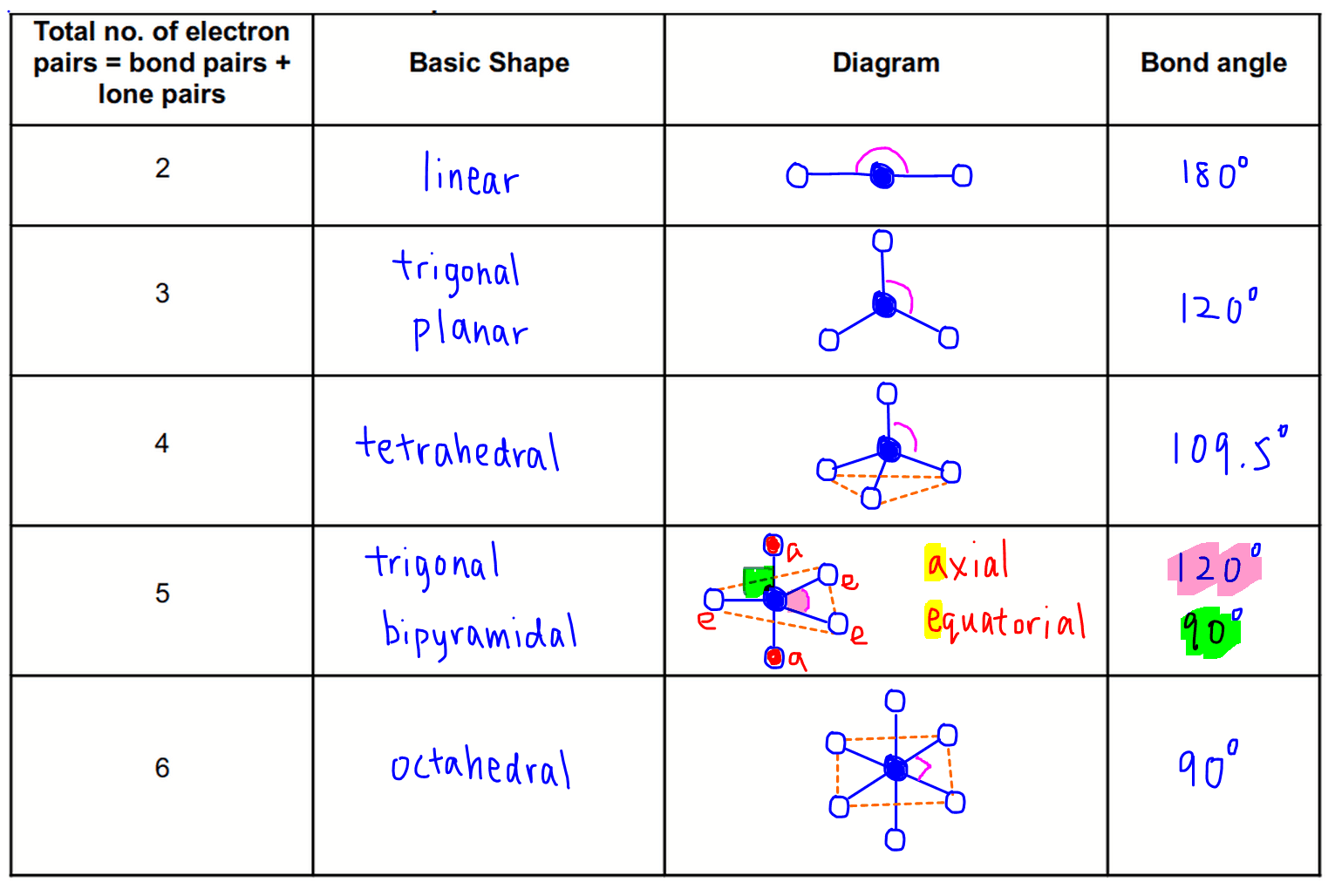
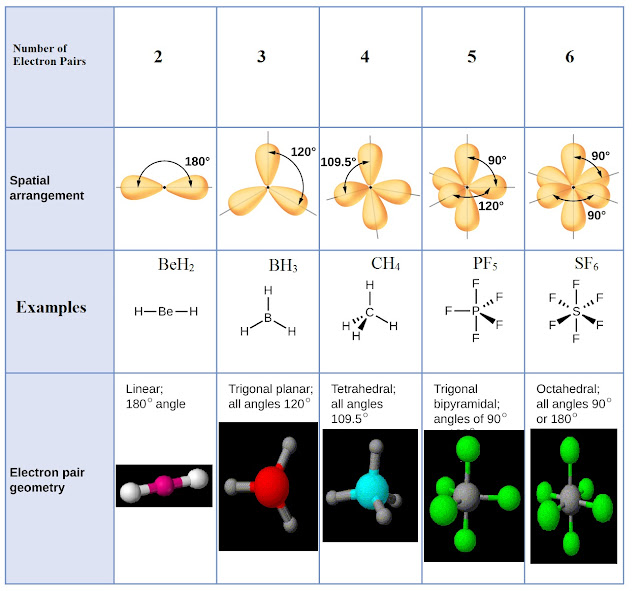
When the two electron groups are 180° apart, the atoms attached to those electron groups are also 180° apart, so the overall molecular shape is linear. A molecule whose central atom contains only two electron groups orients those two groups as far apart from each other as possible-180° apart. Remember that a multiple bond counts as only one electron group.Īny molecule with only two atoms is linear. The VSEPR table below gives you the polarity of molecules of different shapes (assuming that all atoms attached to the central atoms are the same). When applying VSEPR to simple molecules, the first thing to do is to count the number of electron groups around the central atom. There are two types of electron groups: any type of bond-single, double, or triple-and lone electron pairs. shape in which two outside groups are placed. Although C and S have very similar electronegativity values, S is slightly more electronegative than C, and so the C-S bond is just slightly polar. VSEPR makes a distinction between electron group geometry, which expresses how electron groups (bonding and nonbonding electron pairs) are arranged, and. VSEPR theory predicts a linear molecule: The C-O bond is considerably polar. VSEPR makes a distinction between electron group geometry, which expresses how electron groups (bonding and nonbonding electron pairs) are arranged, and molecular geometry, which expresses how the atoms in a molecule are arranged. To determine if this molecule is polar, we draw the molecular structure. It says that electron pairs, being composed of negatively charged particles, repel each other to get as far away from each other as possible. Valence-Shell Electron-Pair Repulsion and the molecular shape.
The basic idea in molecular shapes is called valence shell electron pair repulsion (VSEPR). Small molecules-molecules with a single central atom-have shapes that can be easily predicted. There is an abundance of experimental evidence to that effect-from their physical properties to their chemical reactivity.


 0 kommentar(er)
0 kommentar(er)
